相关文章
The Mystery of Fireworks: What's Inside Fireworks?
Fireworks, an ancient yet modern form of celebration, add infinite splendor to countless festivals and significant moments with their dazzling visual effects. The production of fireworks is a complex art that combines chemistry, physics, and engineering.

A. Chemical Composition of Fireworks
The main ingredient of fireworks is black powder, an ancient chemical mixture composed of potassium nitrate, sulfur, and charcoal. The combination of these three substances gives fireworks their unique burning characteristics.
Potassium nitrate: As an oxidizer, potassium nitrate provides oxygen during the combustion process to help the powder burn. Sulfur: As a combustion agent, sulfur produces a bright blue flame when burning. Charcoal: As a fuel, charcoal releases energy when burning, propelling the fireworks into the air.
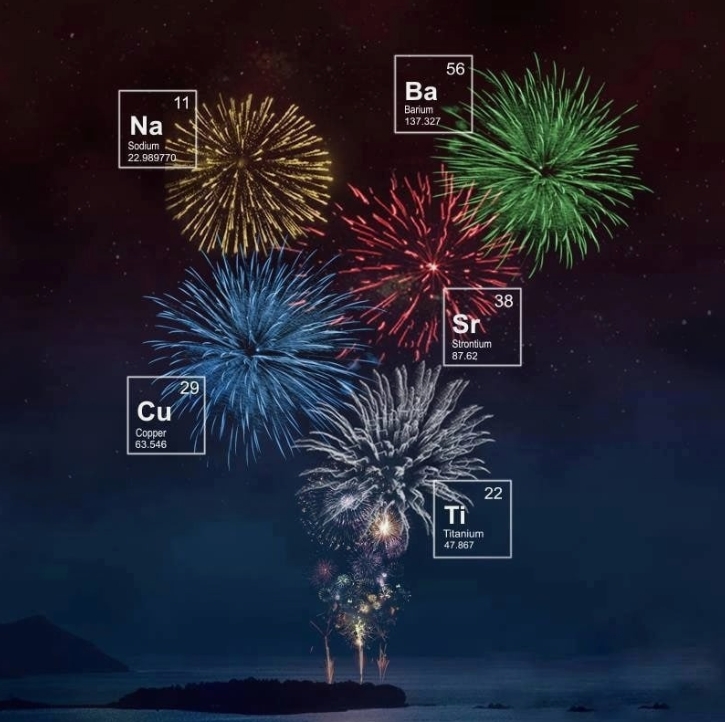
In addition to these three basic components, modern fireworks also add various metal salts to produce different color effects. For example:
Strontium: Produces red flames. Copper: Produces green flames. Barium: Produces yellow or white flames. Aluminum: Produces white flash effects.

B. Physical Structure of Fireworks
The structural design of fireworks is crucial to their effect. A typical firework consists of the following parts:
Shell: Usually made of paper or cardboard, it is used to wrap the powder and other ingredients. Propellant: Located at the bottom of the firework, it is responsible for propelling the firework into the air. Explosive filling: The main body of the firework, containing various colored powders and effect agents. Fuse: The cord that ignites the firework.
C. Working Principle of Fireworks
The working principle of fireworks can be divided into the following steps:
Ignition: When the fuse is lit, the flame moves down the fuse and eventually ignites the propellant. Lift-off: The gas pressure produced by the burning propellant propels the firework into the air. Explosion: After the firework reaches a predetermined height, the explosive filling is ignited, causing an explosion. Colors and effects: The high temperature produced by the explosion causes the metal salts to decompose, releasing different colors of light.
D. Safety and Environmental Protection of Fireworks
The safe use of fireworks is of utmost importance. Manufacturers must ensure that the design of fireworks meets safety standards, including:
Stability: Ensuring the stability of the fireworks during lift-off and explosion. Fuse: Ensuring the length and burning speed of the fuse meet safety requirements. Environmental protection: Using low-pollution chemicals to reduce environmental impact.
Conclusion
Fireworks are not only a way to celebrate, but also a crystallization of chemistry, physics, and engineering. Understanding the composition and working principles of fireworks can help us better appreciate this beautiful art form while ensuring its safety and environmental protection.


